-
- All Centrifuges
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- IVD Products
- High-Speed and Ultracentrifuge Consumables
- Centrifuge Tubes
- Centrifuge Plates
- Device Management Software
- Sample and Information Management
-
- All Pipettes, Dispensers & Automated Liquid Handlers
- Mechanical Pipettes
- Electronic Pipettes
- Multi-Channel Pipettes
- Positive Displacement Pipettes & Dispensers
- Pipette Tips
- Bottle-Top Dispensers
- Pipette Controllers
- Dispenser & Pipette Accessories
- Automated Pipetting
- Automation Consumables
- Automation Accessories
- Liquid Handler & Pipette Services

Automated Bioreactor Sampling with the Bioprocess Autosampler from Eppendorf
Amanda Niles, Sarah Wirth, and Ma Sha Lab Academy
- Bioprocessing
- Automation
- Bioprocess
- Essay
This application note introduces the bioreactor sampling system from Eppendorf and explains how it can increase productivity and efficiency.
Eppendorf Bioprocess Center and Eppendorf, Inc.
In this study we used the Bioprocess Autosampler for automated sampling at various timepoints from four cell culture bioreactors that were operated in parallel.
Key findings:
- Automated bioreactor sampling enhanced productivity by allowing scientists to focus on other tasks while ensuring consistent data collection.
- Automated bioreactor sampling facilitated extended analysis beyond business hours.
- Cell culture metabolites and monoclonal antibodies produced remained stable while being stored in the Bioprocess Autosampler Peltier-cooled sample storage at 4 °C over the weekend.
Read more
Read less
Bioprocess Autosampler: the bioreactor sampling system from Eppendorf
Read more
Read less

Key features
- Enables regular and process-triggered automated sampling and bolus addition 24/7
- Saves space, because no sterile hood is necessary for operation
- Keeps you flexible, because it is compatible with differently sized single-use and glass bioreactors
- Suitable for cell culture and microbial applications
Read more
Read less
How automated bioreactor sampling works with the Bioprocess Autosampler
For this study, the Bioprocess Autosampler was equipped with 4 tools. Each tool holds a 5 mL syringe containing a 23 gauge conical needle. The tools are equipped with a needle guide containing a sterile filter and tubing. This provides a sterile air flow around the needle tip, creating additional protection from contamination. This sterile air flow is automatically activated and controlled by the robot head movements. A multiport module installed on the Bioprocess Autosampler allows for automated sampling and bolus addition to take place (Figure 2). Each multiport module reserves one sample port and one bolus addition port per vessel. These ports contain a septum that provides a sterile boundary to the tubing connected to the bioreactor.
The Bioprocess Autosampler was used in combination with DASGIP Spinner Vessels with pitched-blade impeller (working volume 0.2 to 1 L) that were controlled by the DASGIP Parallel Bioreactor System .
Read more
Read less

A temperature-controlled Peltier-cooled sample storage enables storage of the samples taken, either in 1.5 mL or 10 mL sterilized glass vials. For this study, we used 1.5 mL glass vials to store our samples. The Peltier-cooled sample storage has 3 drawers that holds 2 racks each (6 racks total). A total of 54 samples can be stored in each rack when using the 1.5 mL sized vials. The temperature range for the Peltier-cooled sample storage is 4 °C to 40 °C. For this study, we chose to store our samples at 4 °C.
Bioreactor sampling strategy and scheduling
The Bioprocess Autosampler takes samples in an aseptic operation using a combination of thorough washing cycles to clean the syringe tools as well as dispensing a 70 % ethanol overlay on top of the septum in the sampling port prior to sampling. This layer of ethanol on top of the septum port provides additional protection against contamination.
The Bioprocess Autosampler offers two methods to take a sample: serial and clustered sampling. The serial sampling method uses the same syringe tool to sample each bioreactor (for microbial applications). The clustered sampling method dedicates one syringe tool per bioreactor, significantly lowering the risks of cross contamination between vessels (for cell culture applications and contamination-sensitive microbial applications). The clustered sampling method is the standard method for cell culture use.
Read more
Read less
Samples from all vessels are able to be taken close to each other using the clustered sampling method. The clustered sampling schematic is shown in Figure 3.
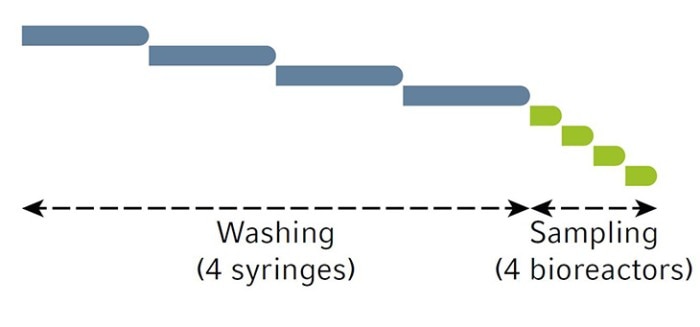
We scheduled the Bioprocess Autosampler to take samples every 12 hours (twice daily) and end sampling after a total of 16 samples per vessel were taken. Our sampling schedule is shown in Figure 4.
Read more
Read less
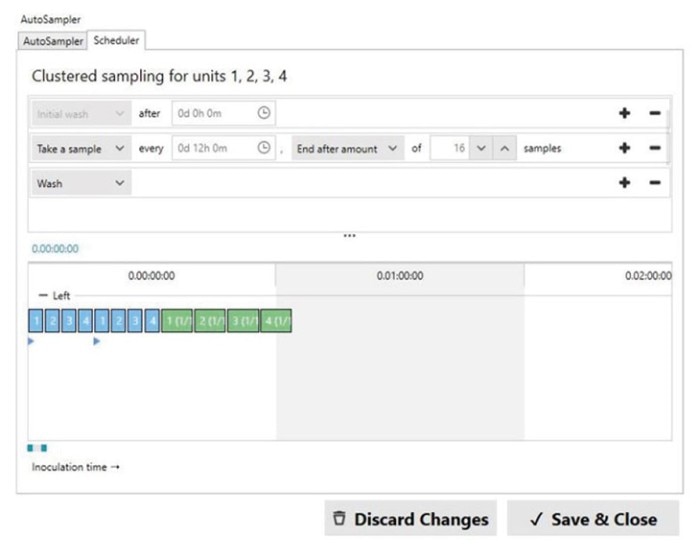
Once the workflow has been started and each vessel has been inoculated, the initial wash and sampling is started. To collect the highest quality sample from the growing culture, the Bioprocess Autosampler takes a dead volume from the sampling line and discards it before taking the actual sample. This dead volume can be adjusted to up to 5 mL. In this study, we used a dead volume of 5 mL. The volume of our taken and stored sample was 1.5 mL.
Read more
Read less
Bioreactor sampling in a CHO cell culture bioprocess
For this study, we used a proprietary suspension CHO cell line from TPG Biologics Inc. that produces the human monoclonal antibody (hMAb) Immunglobulin G (IgG).We cultivated the cells in Dynamis AGT Medium (Thermo Fisher Scientific® ) supplemented with 8 mM GlutaMAX, 1 % antibiotic antimycotic solution, and 1 % Anti-Clumping Agent (Thermo Fisher Scientific) for a complete medium [1].We prepared our cell culture inoculum by cultivating the cells in single-use, baffled bottom shake flasks (VWR) at a 20 % max fill volume. We first thawed the cells from a previously cryopreserved vial using a ThawSTAR® CFT2 Automated Thawing System (BioLife Solutions) and inoculated a 125 mL flask at a seeding density of 0.3 × 106 cells/mL. After that, we incubated the flask in the New Brunswick S41i CO2 Incubator Shaker at 37 °C. Shaking speed was set to 125 rpm and CO2 concentration to 8 %.
Routine passaging and flask culture scale-up
We allowed the freshly seeded cells to grow for a few passages to acclimate them to the new environment prior to scale-up. The CHO cell line’s optimal passage schedule was every other day. After monitoring cell growth and viability, we then started to increase the culture volume from 125 mL to 250 mL, and finally, to 1 L shake flasks. During these scale-up steps, the flask inoculation density, percentage fill and other parameters remained the same [2]. Our target inoculation density for all bioreactors in this study was 0.3 × 106 cells/mL. The shake flask culture was transferred into a 1 L addition bottle that was prepared and sterilized by a steam sterilizer. This addition bottle was then used to inoculate all bioreactors via tube welding (Terumo BCT).
Read more
Read less
Bioprocess parameters for all vessels
When starting a new workflow in the DASware control software, default templates can be used to start and run a process. In this study, we chose to use the default workflow template for cell culture: Cell Cultivation: pH control (CO2/base); DO control (air, pure O2).
For all bioreactors in this study, the dissolved oxygen (DO) was measured using analog DO sensors from Mettler Toledo® . The DO setpoint was set to 50 % for all vessels and controlled at setpoint using 3 - gas auto mixing (Table 1).
Analog pH sensors were used in all vessels for this study. The pH was controlled at setpoint (pH 7.0, deadband ± 0.2) via a cascade to CO2 (acid) and 0.45 M sodium bicarbonate (base). All pH and DO sensors were calibrated as described in the DASware control 6 operating manual [3]. For all vessels in this study, temperature was controlled at 37 °C and remained constant for the duration of the runs (Table 1).
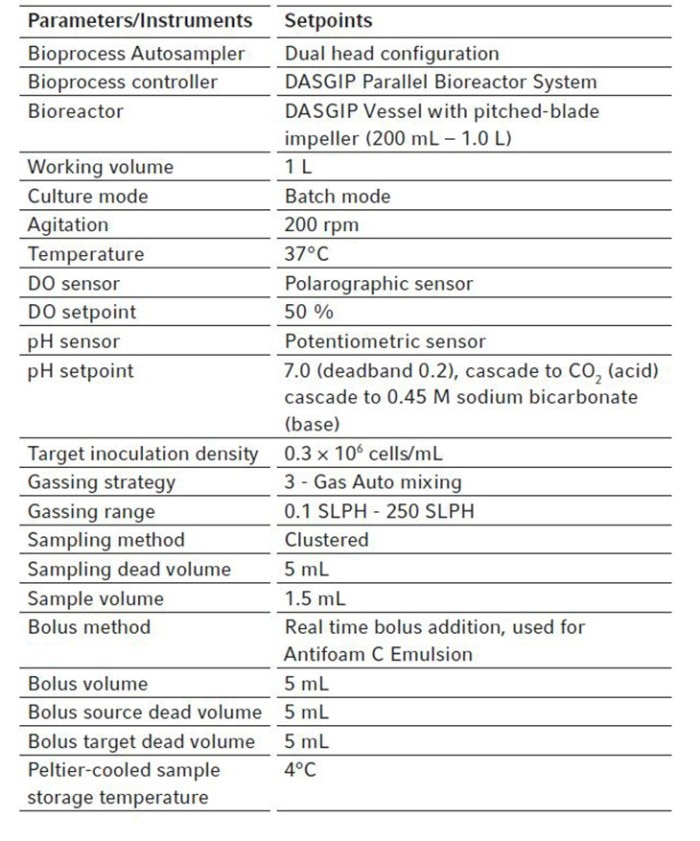
The Bioprocess Autosampler was designed to automate any bolus addition that might be required during a process. With the right scripting, it can be scheduled in accordance with the user’s workflow, based on a specific time or process parameter changes to trigger the bolus addition [2]. However, also real time bolus addition is possible when needed by simply pressing the “add bolus” button under the sampling tab in the DASware control software. In this study, we used the real time bolus addition to add Antifoam Emulsion C (Merck) to our vessels when needed. Once the “add bolus” button is pressed, the bolus addition is scheduled and will start as soon as the Bioprocess Autosampler is free to do so. For our bolus addition, we chose to add 5 mL of Antifoam Emulsion C to all our vessels when needed.
The estimated dead volume of the bolus addition line going into the vessel was 5 mL, which potentially prevents the full bolus volume from reaching the culture. Therefore, after the bolus is added the Bioprocess Autosampler features the function of flushing the tubing with sterile air in order to push the remaining dead volume out of the tubing and into the bioreactor, thus ensuring consistency with each bolus addition step. This way, the more precise automatic bolus addition saves on costly additives, like activating or growth factors. Lastly, the scripting opportunity enables fully automated and situation-specific treatment of the cells, minimizing the need for manual work or being present in the lab at non-working hours.
Automated sampling and analytics
Two samples were taken automatically from each bioreactor daily by the Bioprocess Autosampler, one in the morning and one in the evening. During the week, the samples were stored in the Peltier-cooled sample storage at 4 °C for 24 hours before being analyzed. Samples that were taken over the weekend were stored in the Peltier-cooled sample storage at 4 °C for 72 hours before being analyzed. The cell density, viability, metabolites, biproducts (NH3) and hMAb concentrations were measured.
We measured cell density and viability (via the trypan blue exclusion method) using a Vi-CELL® XR Viability Analyzer (Beckman Coulter). We confirmed our pH values offline using an Orion Star 8211 pH-meter (Thermo Fisher Scientific). If necessary, we used the offline pH measurement to re-standardize the pH calibration on the controller, preventing any discrepancies between online and offline measurements. We measured glucose, ammonia, lactate, and hMAb IgG using a CEDEX® Bio Analyzer (Roche) [1].
Read more
Read less
Results: analysis of bioreactor samples
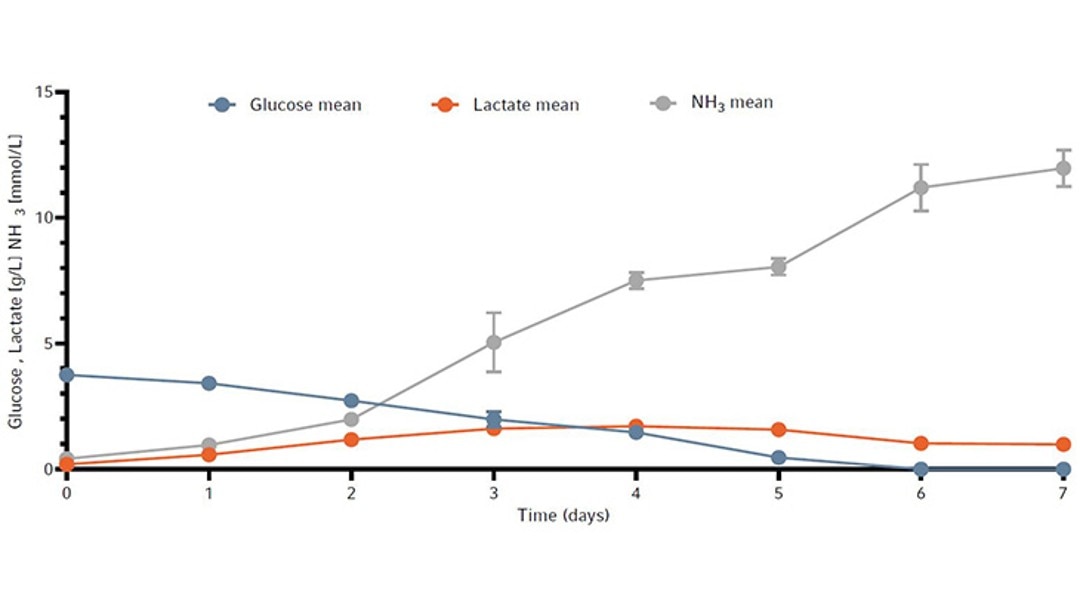
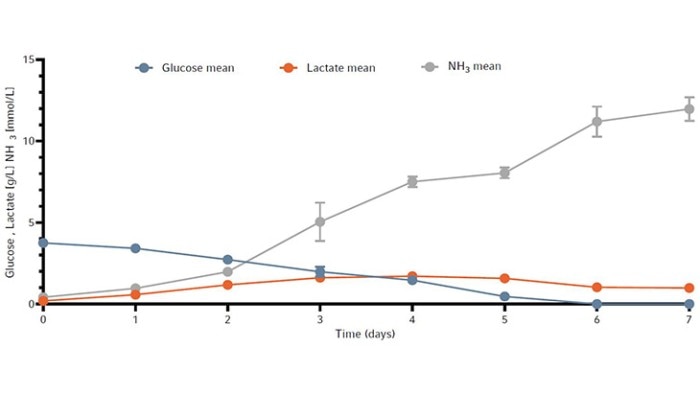
The metabolic profile of the cell culture from all four bioreactors is shown in Figure 5 as the mean +/- standard deviation. Cells were actively growing and depleted initially supplied glucose by day 6. Lactate levels remained under 2 g/L for the duration of the run. Ammonia increased every day to a toxic level of 10.9 mmol/L by day 7 (Figure 5).
Cell growth comparisons
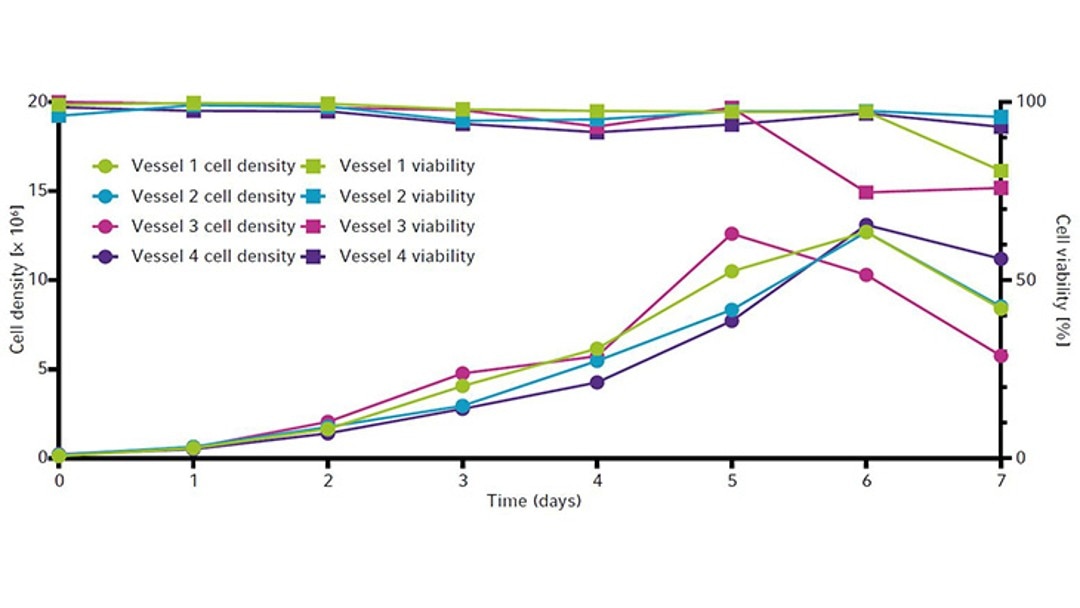
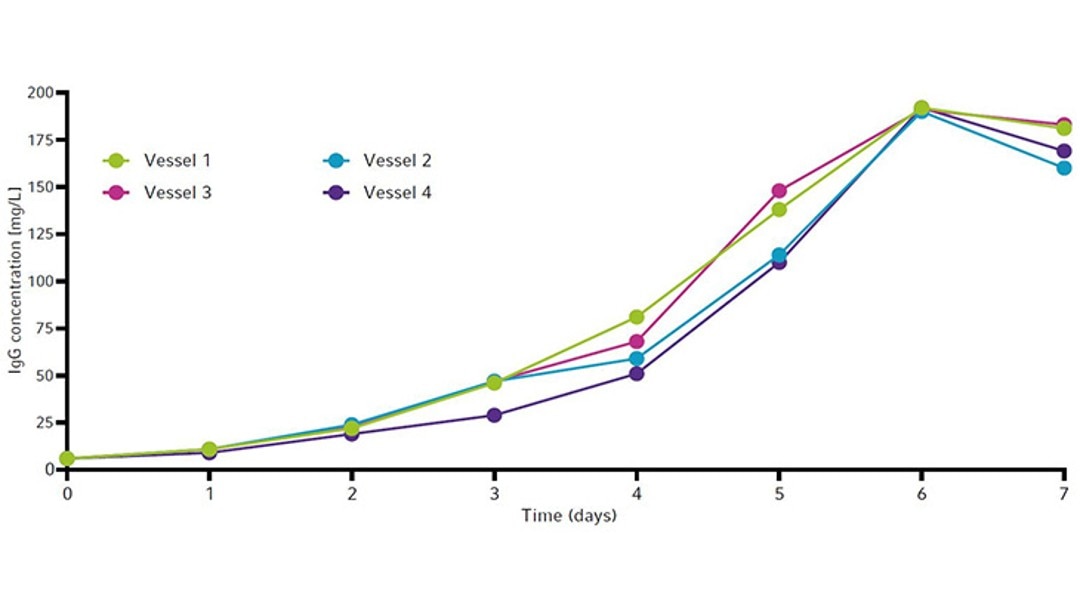
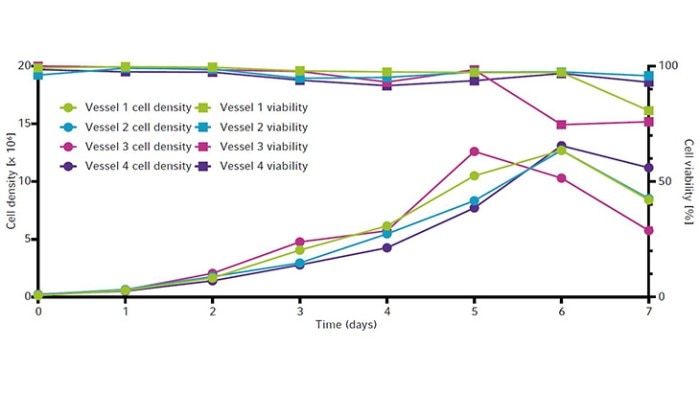
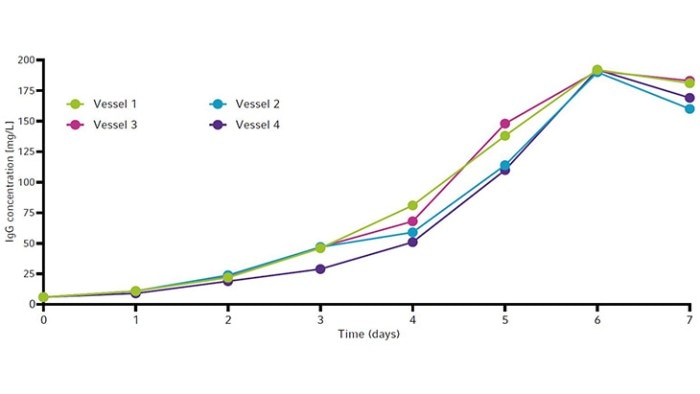
The cell growth curves and viability for all four vessels are shown in Figure 6. All vessels peaked around 13 × 106 cells/mL between day 5 and 6. Viability remained high (90 % or above) until day 5 (Figure 6) before ammonia reached toxic levels (Figure 5). All vessels showed similar IgG yields by the end of the runs. Each culture produced a maximum of around 190 mg/L of IgG by day 6 (Figure 7).
Read more
Read less
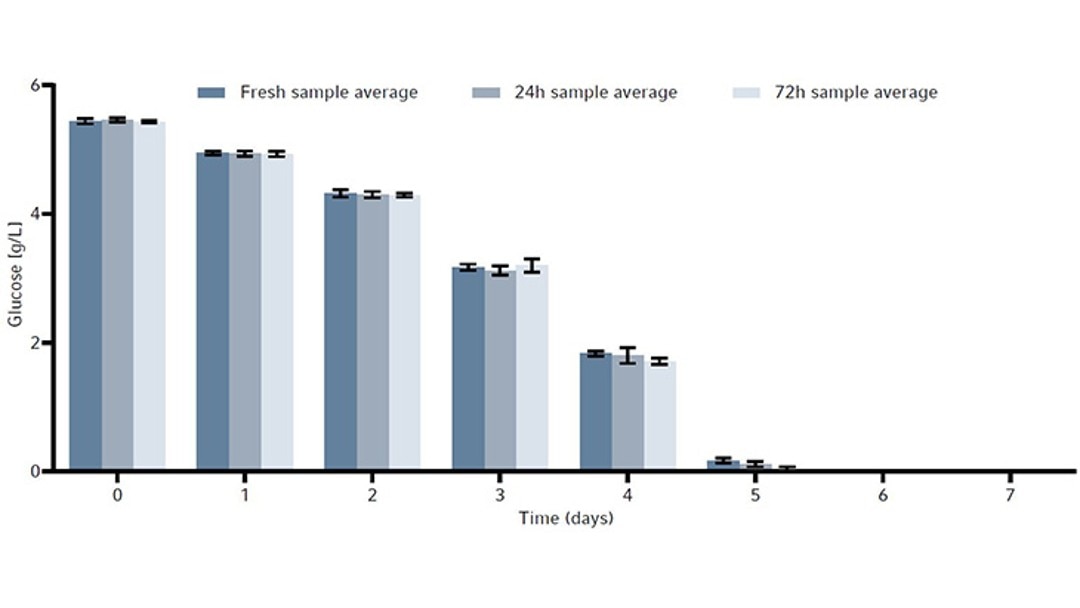
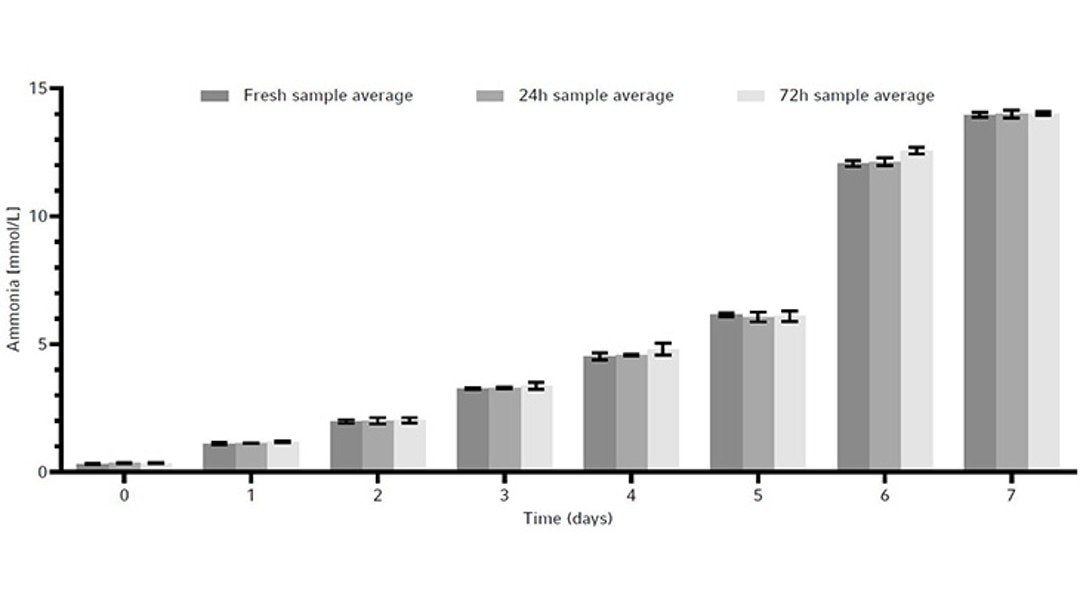
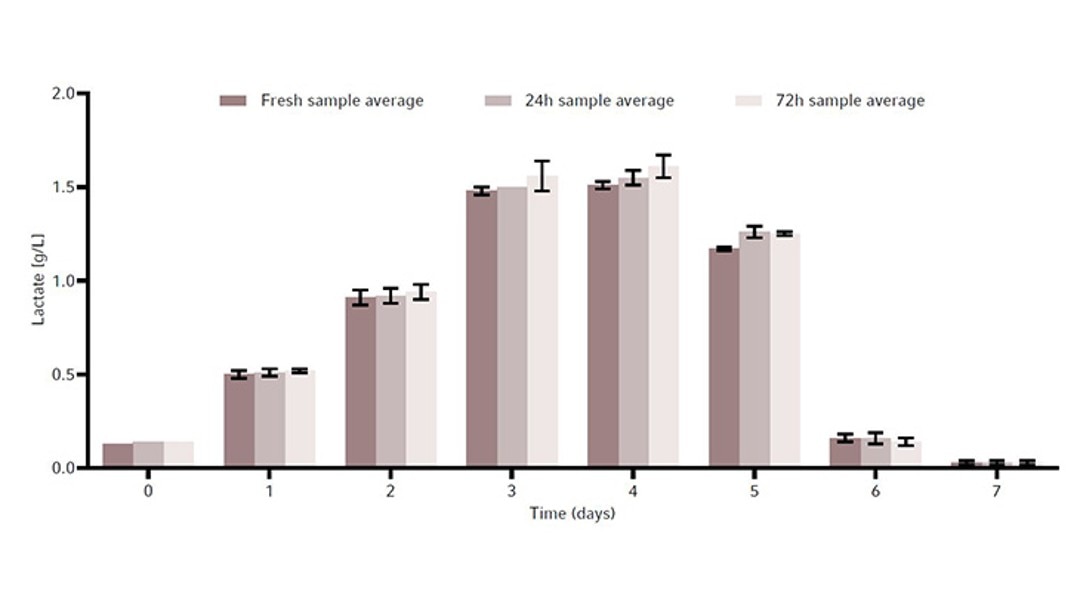
To determine whether sample storage temperature would influence metabolite readings and the stability of the CHO cell line products, a metabolite and product stability study was done by storing samples in the Peltier-cooled sample storage of the Bioprocess Autosampler at 4 °C. For that, the same CHO cell line was grown in a total of 3 × 1 L baffled bottom Erlenmeyer shake flasks in a New Brunswick S41i CO2 Incubator Shaker for 7 days as a batch culture. The reason for the use of Erlenmeyer shake flasks was to demonstrate an easy-to-perfom test for users to evaluate if the Bioprocess Autosampler fits their needs. With that simple procedure the customer can determine prior to purchasing the device whether their cell line or cell products are suitable for storage at 4 °C for maximum 72 hours, for example by storing in a refrigerator.Each 1 L flask was inoculated at 0.3 × 106 cells/mL. We took a 15 mL sample from each flask every day. The 15 mL sample was then split into three 15 mL conical tubes to a final sample volume of 5 mL per tube. These conical tubes were then used to measure the following timepoints: fresh sample, or samples stored in the Bioprocess Autosampler’s Peltier-cooled sample storage at 4 °C for either 24 hours or 72 hours. All fresh samples were frozen immediately at -80 °C, while the others were frozen after the Peltier-cooled sample storage storage period.
The metabolites glucose, ammonia, and lactate, as well as the produced human monoclonal antibody (hMAb) . Immunoglobulin G (IgG) were measured. Metabolites and antibodies were analyzed at the same time by thawing the previously frozen samples and then measuring by using the CEDEX Bio Analyzer. This resulted in three data points per timepoint. Figures 8-11 show the average metabolite or antibody concentration +/- standard deviation. All metabolites and antibodies analyzed resulted in stable measurements, providing confidence in sample storage.
Read more
Read less
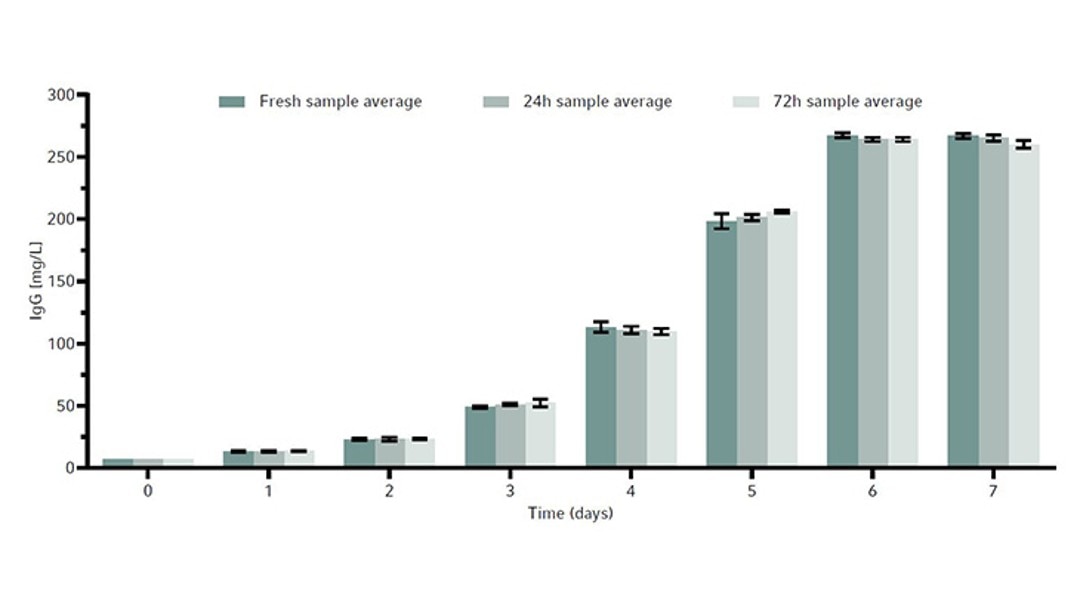
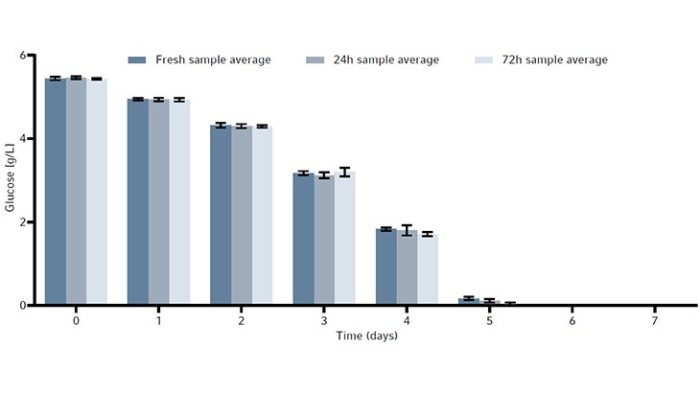
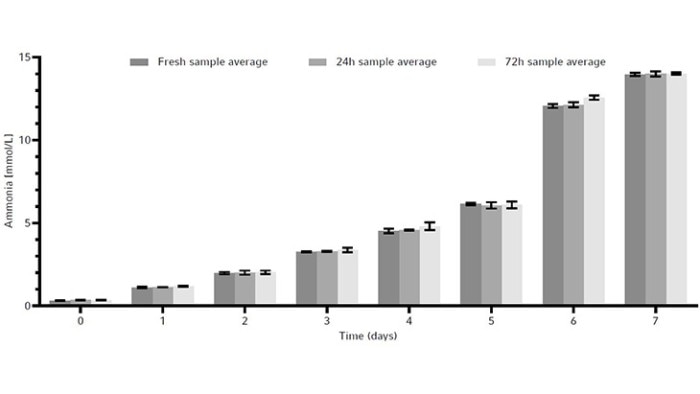
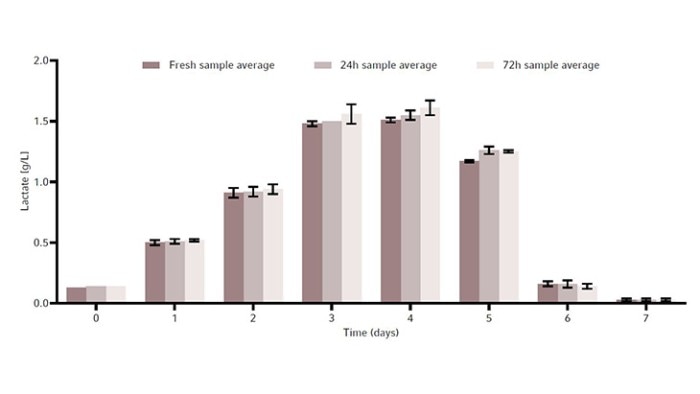
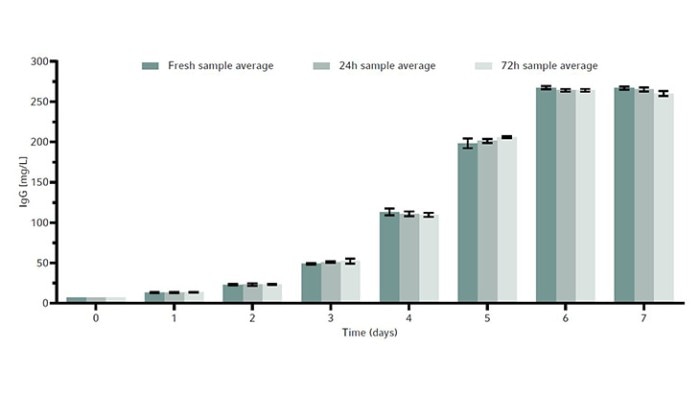
Discussion
We demonstrated how the DASGIP Parallel Bioreactor System in combination with the Eppendorf Bioprocess Autosampler can increase productivity and efficiency, eliminating the need for manual sampling altogether. In this study, we were able to achieve a peak cell density of 13 × 106 cells/mL and peak IgG production of 192 mg/L by day 6 of culture. The cell culture metabolites and monoclonal antibodies that were produced in this study remained stable during 24 and 72 hour-storage in the Bioprocess Autosampler Peltier-cooled sample storage at 4 °C. Furthermore, the Bioprocess Autosampler’s seamless integration into DASware control 6 software allows for ease of use.
Taken together, the automated sampling and bolus additions capabilities greatly increase productivity by reducing the manual workload, freeing up time to spend on other tasks while still expanding critical insights in the process.
Read more
Read less
Literature
[2] Bioprocess Autosampler Operating Manual (2024). Eppendorf, Inc.
[3] DASware Control 6 Operating Manual (2024). Eppendorf, Inc.
Read more
Read less
Read more
Read less


